
In the December 2011 issue of Canadian Chiropractor magazine, Part 1 of this article focused on the role of telomeres in aging
In the December 2011 issue of Canadian Chiropractor magazine, Part 1 of this article focused on the role of telomeres in aging. In Part 2, we continue our investigation of aging and longevity with a look at other factors influencing the aging process and what we can do about them.
 |
|
| In the 1600s, the average human life expectancy was about 30 years. In 2011, the average human life expectancy in Canada is 81. Advertisement
|
WHY DO WE AGE?
Not Just Telomeres
Although intriguing, animal models suggest the association between telomeres and aging is not so simple. Mice, which live only three to four years, have telomeres that are longer than some much longer-lived species, including humans. Nobody knows why, though one possibility is that telomere length is correlated with a much shorter reproductive cycle and mice simply cycle through their telomeres more quickly.1 It is, however, suggestive that telomeres alone do not determine lifespan.
“Once a person is older than 60, their risk of death doubles with every eight years of age. So a 68-year-old has twice the chance of dying within a year compared with a 60-year-old. [One study] found that differences in telomere length accounted for only four per cent of that difference and only another six per cent is due purely to chronological age. When telomere length, chronological age and gender are combined … those factors account for 37 per cent of the variation in the risk of dying over age 60. So what causes the other 63 per cent?”2
This is where the damage or error theories of aging kick in. They include factors such as: rate of living, wherein the greater an organism’s rate of oxygen basal metabolism, the shorter its life span; crosslinking, an accumulation of crosslinked proteins that damage cells and tissues; and somatic DNA damage, the accumulation of genetic mutations with increasing age, causing cells to deteriorate and malfunction.3
OXYGEN RADICALS
Another error theory – one which has attracted a lot of attention lately – involves the accumulated damage caused by oxygen radicals. A radical is an atom or group of atoms that have one or more unpaired electrons. According to the free radical theory of aging, damage caused by oxygen radicals is responsible for many of the bodily changes that characterize senescence. This “oxidative stress” is the damage to proteins, membranes, and nucleic acids, particularly DNA caused by oxidants. Oxidants are highly reactive substances containing oxygen-free radicals. These oxidants are created in the normal course of metabolism, produced as cells turn food and oxygen into energy. Free radicals also result from inflammation, infection, and consumption of alcohol and cigarettes, and have been implicated in a variety of degenerative disorders, including cancer, atherosclerosis, cataracts and neurodegeneration.4, 5
To defend against these damaging molecules, our bodies employ a suite of antioxidants, which our cells use to neutralize oxygen radicals. Antioxidants include vitamins C and E and beta carotene as well as enzymes, such as superoxide dismutase (SOD), catalase and glutathione peroxidase. They mitigate but cannot prevent all oxidative damage. Over time, the accumulated damage leads to cellular senescence and apoptosis, a form of programmed cellular death. Increasing damage contributes, so the theory goes, to deteriorating tissues and organs.6
The free radical theory is supported by antioxidant research, particularly with regard to SOD. SOD is a key component in the process of converting destructive oxygen radicals into harmless oxygen and water.
“Studies have shown that inserting extra copies of the SOD gene into fruit flies extends their average lifespan by as much as 30 per cent.”7 Other research found that by giving worms two substances that neutralize oxidants, the worms’ lifespan improved an average 44 per cent.8 Also suggestive of the importance of combating oxidative stress is the finding that longer-lived animals have higher levels of SOD. SOD levels have been directly related to life span in 20 different species. Levels of other antioxidants have also been correlated with life span.9
GLYCATION
Another major agent in aging is glucose – blood sugar. Glucose sugar molecules from what we eat attach themselves to proteins, initiating a domino-like sequence of chemical reactions that causes proteins to bind together, or crosslink. Called non-enzymatic glycosylation, or glycation, this process can fundamentally alter the proteins’ biological and structural roles. Crosslinked proteins accumulate over time and eventually disrupt cellular function, causing intracellular damage and apoptosis. Body tissues malfunction, resulting in disease and death.
These crosslinks, also known as advanced glycosylation end products (AGEs), toughen tissues and may be responsible for some of the wear and tear associated with aging. AGEs have been related to stiffening collagen, a normally pliable protein, with the result that lungs, arteries, tendons and other tissues lose flexibility and become less efficient. They have been implicated in the progression of a variety of age- and diabetes-related chronic diseases, including atherosclerosis, cardiovascular disease and stroke, development of cataracts, nephropathy and neurological disorders such as Alzheimer’s disease.10, 11 In addition, free radicals created through oxidation- and glycation-instigated crosslinks appear to accelerate the formation of one another.
The relationship between glycation and diabetes has led some to consider diabetes an accelerated model of aging.
“Not only do the complications of diabetes mimic the physiologic changes that can accompany old age, but people with this condition have shorter-than-average life expectancies. As a result, much research on crosslinking has focused on its relationship to diabetes as well as aging.”12
EXTENDING LONGEVITY
In the 1600s, the average human life expectancy was about 30 years.13 In 2011, the average Canadian life expectancy is 81.14 Much of the increase in life expectancy is accounted for through improved nutrition, modern concepts of sanitation, clean water, refrigeration, antibiotics, vaccines and other medical advances. Now research is underway to not only radically further extend life expectancy but also perhaps extend the biological limits of how long a human being can live – our life span.
CALORIC RESTRICTION
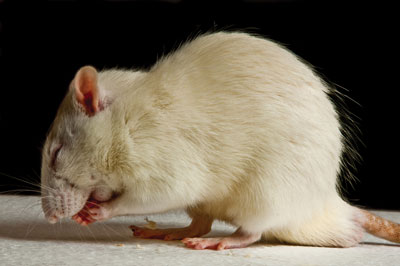
In the recent stampede towards longevity research, perhaps the single most important observation was made in 1935 when a Cornell University nutritionist reported that feeding rats a near starvation diet extended their life span by as much as 50 per cent – a finding that was replicated in mice several years later.15 Feeding these animals a diet that had at least 30 per cent fewer calories than they would normally consume also dramatically inhibited tumours. They not only lived longer but were more active and showed less pathology in the heart, kidneys, liver and other organs.16 These studies, building on research in the early 1900s, were the first to demonstrate that a reduction in food intake was capable of increasing life span and inhibiting tumour formation in at least some animals.17
This research went largely unnoticed until the underlying genes were discovered in the early 1990s. Their discovery ignited the current explosion of life-extension research. “The effect of calorie restriction on health and longevity has been shown to hold true not just for rodents but also for yeast, protozoa, fruit flies, worms, spiders and perhaps monkeys. The intervention prevents heart disease, cancer, diabetes, kidney disease, cataracts, Parkinson’s and Alzheimer’s. It improves cholesterol profiles, lowers blood pressure, and prevents the deterioration of the immune system that naturally accompanies aging.” 18 In fact, animals on calorie-restricted diets not only consistently exhibit reduced rates of disease but caloric restriction also appears to delay normal age-related degeneration of almost all physiological systems, including intellectual function.19 What remains to be seen is whether the same health and longevity benefits can be reproduced in primates, humans among them. Research taking place today has yielded some promising preliminary trends, including the possibility of reduced incidence of heart disease and cancer among calorically restricted monkeys. However it is still too early to know if caloric restriction actually improves the health and extends the life span of aging primates.
 |
|
| Animals on calorie-restricted diets may show reduced rates of disease and delayed age-related degeneration.
|
Ethical issues aside, experimentation in humans is not possible because we typically live much longer than rats or mice. Caloric restriction has, however, been shown to improve biomarkers of health status such as blood pressure and cholesterol profiles in humans, at least over the short term of the studies. The obvious limits to this type of research are that few of us would willingly subject ourselves to the severe and likely unpalatable regimen of lifelong privation that a diet of 30 per cent fewer calories would require – particularly when long-term benefits remain to be proven in humans. Nonetheless, some believe caloric restriction “remains the only known behavioral intervention capable of delaying the onset of many age-related diseases and extending maximal longevity.” 20
HOW DOES CALORIC RESTRICTION WORK?
We do not know why caloric restriction appears to deliver the longevity and health dividends that it does though several theories have been proposed. The explanation that has stood up best to date has to do with caloric restriction’s capacity to influence the secretion of insulin and insulin-like growth factor (IGF) hormones. Although the underlying mechanisms of longevity are not fully understood, it is known that mutation in genes that share similarities with those of humans involved in the insulin/IGF signal response pathway can significantly extend life span. Particularly long-lived individual organisms exhibit some key common phenotypic characteristics, such as reduced insulin signalling, enhanced sensitivity to insulin, and lowered IGF plasma.21 IGF hormones “signal organisms to channel their resources into either growth and reproduction (when insulin and IGF levels are high) or maintenance and repair (when they are low)” and respond to the presence or absence of food.22 In other words, they respond to how much we eat, particularly of sugars and starches, and regulate metabolism, fat storage, and reproduction.
While IGF promotes cell division and growth, insulin channels food energy either for immediate use or into storage. In times of plenty, insulin and IGF levels increase and signal organisms to grow, mature and reproduce. When food is scarce, insulin and IGF levels fall. “Activity in the insulin/IGF signaling pathway is reduced, and the animal shifts into a maintenance mode that favors long-term survival over immediate reproduction. The outcome is a redirection of resources toward repairing and protecting cells.”23
A food-sensing mechanism of this type has clear adaptive implications. Regulation of metabolism, food utilization pathways and life span all serve a similar biological function: to allow animals to postpone reproduction during unfavourable environmental conditions. Such a system would prompt animals to build reserves when resources are scarce and curtail reproduction until food is plentiful. Organisms lacking such a system would either die of starvation or produce offspring in times of scarcity, both limiting their chances for survival and increasing competition for limited resources. “It also activates pathways that extend life span, which increases the organism’s chance of being alive and still youthful enough to reproduce if it takes a long time for conditions to improve.”24
A CROWDED LANDSCAPE
Aging is a complex mosaic of interacting processes “potentially involving every molecule, cell and organ in the body.”25 At a minimum, factors such as telomere shortening, oxidative stress, glycation and chronological age – along with various genes – all work together to cause aging. It is a landscape crowded with factors and processes that lead to what has, until recently, been seen as our inevitable decline.
The fundamental relationship between many of these age-related biochemical changes to the health of individual patients and to the morbidity associated with aging in the population at large are examined in the undergraduate curriculum at the Canadian Memorial Chiropractic College (CMCC). According to Dr. Marion McGregor, Director, Education Year 2, at CMCC, “The chiropractic profession, like all health professions, is grounded by the first principles in basic sciences. From the basics in physiology, anatomy and pathology, clinical decisions are made, and as such the biochemistry courses in the undergraduate curriculum include topics like glycation and oxidative stress. Even caloric restriction is touched on, though greater emphasis is placed on the impact of excessive nutrition. Chiropractors remain primary contact providers and when discoveries are made in arenas that impact an already aging population, the chiropractic community, like the student body, takes notice of emerging work that may affect the health of society at large.”
In Part 3, we will cross paths with the insulin/IGF signalling pathway again when we look at the genetic component behind the extreme longevity of the longest-lived among us. We will also explore the chiropractor’s role in how wellness care and mind-body relationships relate to an individual’s health span as opposed to their life span.
REFERENCES
- In conversation with G. Sovak, PhD (September, 2011).
- Siegel, LJ. Are telomeres the key to aging and cancer? Genetic Science Learning Center. University of Utah. Available from: http://learn.genetics.utah.edu/content/begin/traits/telomeres
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 6.
- Siegel, LJ. Are telomeres the key to aging and cancer? Genetic Science Learning Center. University of Utah. Available from: http://learn.genetics.utah.edu/content/begin/traits/telomeres
- National Institute on Aging, National Institutes of Health (US). In search of the secrets of aging: biochemistry and aging. NIH Pub. No. 93-2756, 2011.
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756.: 19.
- Ibid: 20.
- Siegel, LJ. Are telomeres the key to aging and cancer? Genetic Science Learning Center. University of Utah. Available from: http://learn.genetics.utah.edu/content/begin/traits/telomeres
- National Institute on Aging, National Institutes of Health (US). In search of the secrets of aging: biochemistry and aging. NIH Pub. No. 93-2756, 2011.
- Peppa, M et al. Glucose, advanced glycation end products, and diabetes complications: what is new and what works. Clinical Diabetes. 2003 Oct 21(4):186-187.
- Nass N, Bartling B, Navarrete Santos A, Scheubel RJ, Börgermann J, Silber RE, Simm A. Z Gerontol Geriatr. 2007 Oct; 40(5):349-56. Review.
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 20.
- Siegel, LJ. Are telomeres the key to aging and cancer? Genetic Science Learning Center. University of Utah. Available from: http://learn.genetics.utah.edu/content/begin/traits/telomeres
- Statistics Canada, CANSIM, table 102-0512 and Catalogue no. 84-537-XIE. 2011 [last modified: 2011-09-27]. Available from: http://www40.statcan.ca/l01/cst01/health26-eng.htm
- Taubes, G. The timeless and trendy effort to find – or create – the Fountain of Youth. Discover Magazine. Oct 2010. Available from: http://discovermagazine.com/2010/oct/12-timeless-trendy-effort-find-create-fountain-youth
- Tannenbaum, A. Nutrition Classics: The American Journal of Cancer, Volume XXXVIII, Mar 1940: The initiation and growth of tumours. Introduction 1. Effects of underfeeding. By Albert Tannenbaum. Nutrition Review. 1987; 45: 20-22.
- Huffman, DM, Barzilai, N. Contribution of adipose tissue to health span and longevity. Body composition and aging/volume editors, CV Mobbs, PR Hof. Interdisciplinary topics in gerontology. 2010; 37: 12.
- Taubes, G. The timeless and trendy effort to find – or create – the Fountain of Youth. Discover Magazine. Oct 2010. Available from: http://discovermagazine.com/2010/oct/12-timeless-trendy-effort-find-create-fountain-youth
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756.: 35.
- Huffman, DM, Barzilai, N. Contribution of adipose tissue to health span and longevity. Body composition and aging/volume editors, CV Mobbs, PR Hof. Interdisciplinary topics in gerontology. 2010; 37: 12.
- Barbieri, M et al. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. AJP-Endocrinol Metab. 2003 Nov; 285: E1064.
- Taubes, G. The timeless and trendy effort to find – or create – the Fountain of Youth. Discover Magazine. Oct 2010. Available from: http://discovermagazine.com/2010/oct/12-timeless-trendy-effort-find-create-fountain-youth
- Ibid.
- Ibid.
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 3.
Steve Zoltai is the collections development librarian and archivist for CMCC and is a member of the Canadian Chiropractic Historical Association. He was previously the assistant executive director of the Health Sciences Information Consortium of Toronto. He has worked for several public and private libraries and with the University of Toronto Archives. Steve comes by his interest in things historical honestly – he worked as a field archeologist for the Province of Manitoba. He can be contacted at szoltai@cmcc.ca.
Print this page