
Microbiome balance: The importance of gastrointestinal health for thyroid hormone conversion
By Dr. Whitney Baxter, ND
Features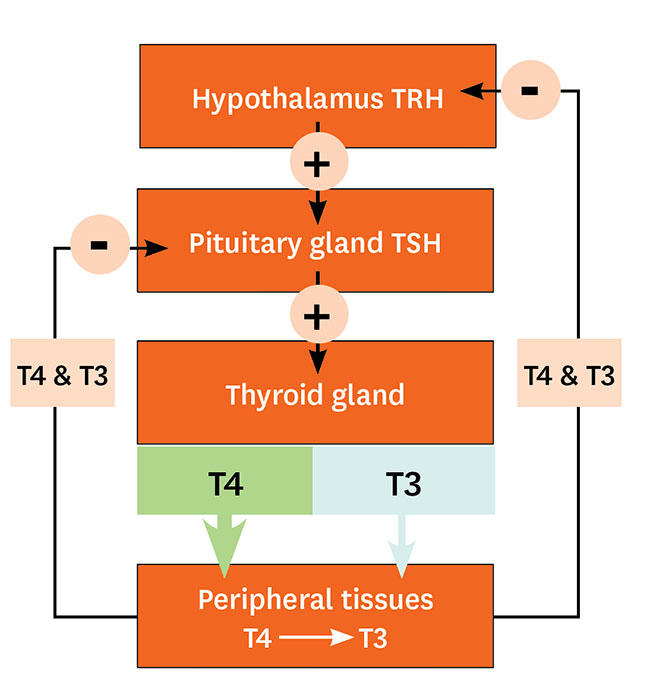 An overview of hypothalamic-pituitary-thyroid axis showing the feedback system of circulating thyroid hormone.1
An overview of hypothalamic-pituitary-thyroid axis showing the feedback system of circulating thyroid hormone.1 Naturopathic doctors often look to the gastrointestinal tract (GIT) for the root cause of conditions. A major link is the microbiome, the vast ecosystem of microbes existing mainly in the large intestine, that is important to our physiology for immune surveillance, digestion, and nutrient absorption. Altered intestinal microbial balance, commonly known as dysbiosis, impacts these important functions and has been implicated in conditions such as autoimmune diseases, depression, heart disease, skin reactions and nutrient deficiencies.
Underlying causes of hypothyroidism is no exception here. Studies are beginning to show the role of dysbiosis in decreased thyroid hormone synthesis, decreased active thyroid hormone conversion and autoimmune Hashimoto’s Thyroiditis (HT).1
This article explores the evidence linking dysbiosis with hypothyroid conditions and the importance of this link when treating patients presenting with symptoms of hypothyroid.
Thyroid physiology
The thyroid controls the metabolic rate of every cell. It is important for growth regulation, cell differentiation and energy generation from the foods we eat.
Thyroid hormone production depends on the organized feedback system that involves thyroid stimulating hormone (TSH) release from the pituitary, stimulating the synthesis and release of thyroxine (T4) and some triiodothyronine (T3 , the active form). Most of this T3 conversion occurs in the peripheral tissues via the deiodinase system of enzymes.2
There are a few key nutrients involved along the way: iodine and iron are essential in the synthesis of T4 and T3, and selenium and zinc are crucial to multiple steps, especially in the conversion of T4 to active T3.
How the microbiome influences thyroid function
Researchers are starting to recognize this relationship and noticing a number of mechanisms that are briefly introduced here. First, the microbiome is vital to the absorption of nutrients required for thyroid hormone synthesis and T3 conversion. Second, seventy percent of our immune system exists along the GIT, so it makes sense that the microbiome shares a close symbiotic relationship with our immune system.3 This is important for regulating inflammation and appropriate immune responses. Dysfunction in this relationship has been shown to be a factor in the development of autoimmune diseases, including HT.4 And finally, the microbiome plays a role in deiodinase-dependent T3 to T4 conversion and of their metabolism in the intestines.5
Here is a closer look at the interaction of the nutrients outlined above with the microbiome:
- Iron: beneficial microbial strains, specifically Lactobacilli and Bifidobacterium, increase iron absorption by altering the pH of the colon via the production of short chain fatty acids (by-products of these strains).3,6. On the other hand, pathogenic bacteria utilize iron for themselves as a fuel source, resulting in decreased availability of iron for thyroid hormone production.
- Iodine: iodine is absorbed mainly in the stomach, small intestine and to a lesser extent in the large intestine. Inflammation or dysbiosis in these upper portions of the GIT, such as IBD and gastritis, has been shown to reduce iodine absorption.1,7
- Zinc and Selenium: both of these minerals have been shown to positively influence beneficial strains of the microbiome.1
The relationship between the microbiome and thyroid function has been demonstrated in both mouse and human studies. One study showed that when mice were given probiotics with Lactobacillus reuterii species, there was increased thyroid function (higher levels of T4, larger thyroid gland mass, more active behaviour and thinner shape). 3
Probiotics are often used in addition to a gut healing protocol in patients with dysbiosis or other causes of GIT inflammation. More human studies are needed to better understand their specific therapeutic benefit for thyroid diseases and absorption of important minerals for T3 conversion. An outline on how naturopathic doctors approach an individualized GIT and thyroid treatment plan is provided below.
Autoimmune disease and dysbiosis
It has long been recognized that impaired GIT integrity (increased permeability or “leaky gut”) and dysbiosis is correlated with autoimmune conditions including those of the GIT (IBD and Celiac) and thyroid (both hyper or Grave’s Disease and hypo, HT).
HT is a major cause of hypothyroidism, characterized by circulating T cells and antibodies against the thyroid gland (specifically reacting to thyroid peroxidase and thyroglobulin required for making thyroid hormone).1Different compositions of microflora are seen in those with autoimmune hypothyroidism (HT) and studies are beginning to discover potential mechanisms of disease etiology including self-antigen formation, reduced immune tolerance and production of inflammatory lipopolysaccharide (LPS) from gram negative bacteria.5
Approach to healing the GIT, microbiome and thyroid function
When patients present with hypothyroid symptoms, a thorough assessment is done in order to determine underlying causes of thyroid dysfunction (symptom history, pertinent blood work and physical exams). Underlying GIT inflammation or dysbiosis should also be investigated in the presence of digestive symptoms (indigestion, bloating, IBS-like symptoms, food sensitivities, generalized fatigue or nutrient malabsorption) or with comprehensive DNA microbial testing (stool testing).
Therapies to support thyroid function include addressing nutrient status (iodine, iron, selenium and zinc) and reduction of inflammation either through the diet, supplementation or herbal medicine. If needed, pharmaceutical intervention is considered to return TSH, T4 and T3 levels to their optimal range.
Inflammation and dysbiosis along the GIT is treated by removing any inflammatory triggers (certain foods, long term stress or underlying infection), supporting normal digestive function (digestive enzymes and/or probiotics) and nutrients shown to repair the GIT lining and reduce inflammation (examples include L-glutamine, zinc, demulcent herbs, adequate hydration and fiber).
Each presenting case of hypothyroidism is unique, and with this comprehensive approach, any links between GIT health, dysbiosis and thyroid function can be discovered and supported.
REFERENCES:
- Fröhlich, E. and Wahl, R. (2019). Microbiota and Thyroid Interaction in Health and Disease. Trends in Endocrinology and Metabolism. Cell Press Reviews
- Luongo, C.et al. (2019). Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol15, 479–488. https://doi.org/10.1038/s41574-019-0218-2
- Knezevic, J. et al. (2020). Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function?. Nutrients, 12(6), 1769. https://doi.org/10.3390/nu12061769
- Lerner, A., Jeremias, P., & Matthias, T. (2017). Gut-thyroid axis and celiac disease. Endocrine connections, 6(4), R52–R58. https://doi.org/10.1530/EC-17-0021
- Virili, C., et al. (2018). Gut microbiota and Hashimoto’s thyroiditis. Reviews in Endocrine and Metabolic Disorders. doi:10.1007/s11154-018-9467-y
- Yilmaz, B., & Li, H. (2018). Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals (Basel, Switzerland), 11(4), 98. https://doi.org/10.3390/ph11040098
- Beyer, S. (2016). Gut Microbes, Gut inflammation and your Thyroid. Integrative Brain and Body
Print this page